Transcatheter aortic valve replacement (TAVR) has emerged as a life-saving solution for inoperable elderly patients with end-stage calcific aortic valve (CAV) disease [1]. However, valve migration, paravalvular leakage (PVL), and thrombogenic potential may limit its expansion into younger, lower-risk patients [2]. Previous numerical studies attempted to address these complications but neglected the valve post-deployment performances during heart beating [3]. This study utilizes Simulia Living Heart Human Model (LHHM) heart beating capabilities coupled with a fluid-structure interaction (FSI) simulation to evaluate TAVR complications.
Materials and Methods: LHHM is an electro-mechanical model of heart function in which a self-expandable TAVR valve (Evolut R; Medtronic, Inc.) implantation procedures were simulated using finite element modeling (Abaqus Explicit 6.14; Dassault Systèmes). Three implantation depth locations (aortic, midway, and ventricular) following the manufacturer guidelines were modeled to study the valve anchorage. The anchorage was evaluated based on the calculated contact area between the stent’s outer frame with the native CAV over time [3]. The deformed TAVR stent equipped with the prosthetic leaflets and LHHM anatomy were extracted for FSI simulation to assess and quantify the valve hemodynamics parameter, PVL degree, and thrombogenicity. A 2-way implicit coupling (FlowVision Multi-Physics Manager 3.10) was used between a finite-volume based body-fitted sub-grid geometry resolution method (FlowVision 3.10) and a finite element solver (Abaqus Explicit 6.14) during the FSI analysis fluid and structural solution, respectively.
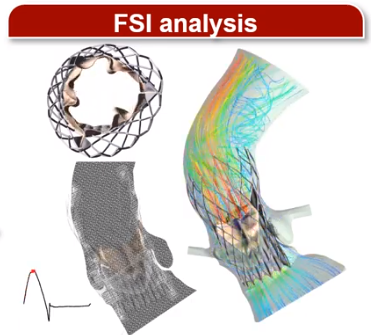
Results and Discussion: Figure 1A, B, and C represent a deployed stent in LHHM, the stent configuration at the end of 3rd cardiac cycle, and the stent anchoring contact area over time, respectively. The optimal stent anchorage was observed when the stent was deployed more towards the ventricular side (Fig. 1B; right) and migration was observed when the implantation was more towards aorta (Fig. 1B; left). Therefore, the TAVR valve configuration from the ventricular positioning was used during the FSI analysis (Fig. 1D). Recirculation zones were observed in the sinuses throughout systole and a helical flow pattern was formed in the aortic arch region during peak systolic phase. During diastole, PVL flow was observed near the commissures. PVL degree and thrombogenicity quantification through PVL gaps are currently being studied to determine the optimal valve positioning.
Fluid-Structure Interaction Analysis of TAVR Valve Performances in Living Heart Human Model
Fluid-Structure Interaction Analysis of TAVR Valve Performances in Living Heart Human Model Video
Ram P. Ghosh1, Gil Marom2, Matteo Bianchi1, Karl D'souza3, Wojtek Zietak4, Danny Bluestein1
1 Stony Brook University, USA; 2 Tel Aviv University, Israel; 3 Dassault Systemès, USA; 4 Capvidia, Belgium.